Advanced Treatment Planning
Proton beams show considerable physical advantages in the field of radiation therapy due to the energy deposition peak at the end of their penetration depth in matter. However, due to several factors such as conversion of computed tomography (CT) data into stopping power ratio (SPR), patient positioning, and anatomical changes, calculations of the beam penetration depth, i.e. proton range, exhibit non-negligible uncertainties. As one of the most promising approaches, prompt gamma (PG) imaging can enable in-vivo monitoring of the proton range by detecting the PG emitted by nuclear de-excitation processes in the beam path.
The accuracy and precision of PG imaging are affected by both tissue heterogeneities and counting statistics. These effects are not considered in current treatment planning systems (TPS), and the intensities of most pencil beams are not optimized with respect to the precision of PG range monitoring and usually no individual pencil beam has enough protons to enable a reliable proton range monitoring.
In our research, we investigate the possibility of re-optimizing TPS accounting for in-vivo proton range monitoring. To this end, Monte Carlo treatment plans are firstly created using a research computational platform, combining Monte Carlo (Geant4) pre-calculated pencil beams with the analytical TPS engine CERR (a Computational Enviroment for Radiotherapy Research). PG generation and dose distribution of all individual pencil beam are simulated using Geant4 and then compared. Next, the conformities of dose and PG profiles are quantified. Finally, new treatment plans (re-optimized plans), in which a few automatically or manually selected spots with the best PG-dose correlation are boosted to ensure PG detectability with good precision, are made.
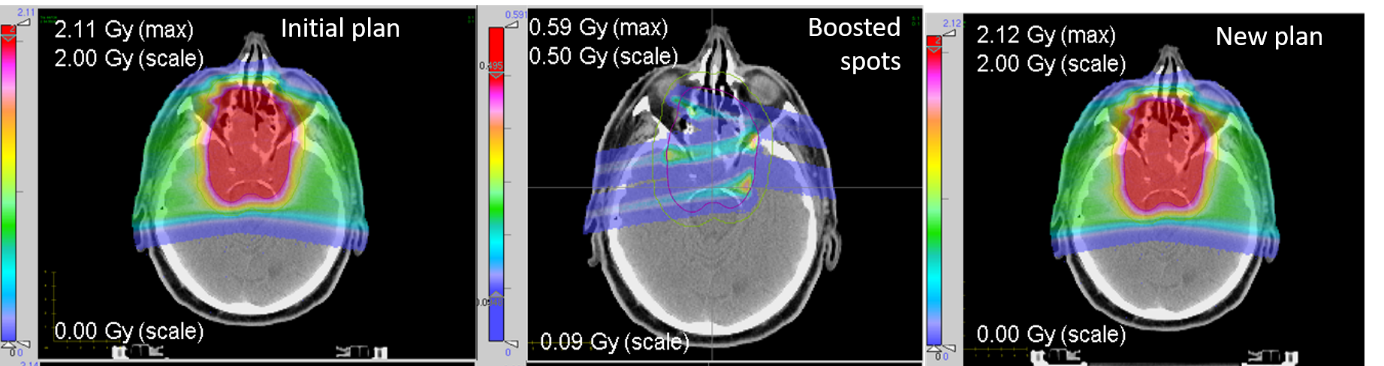
Our first study (see example in figure) showed that the re-optimized plan can be comparable to the initial plan in terms of dose distribution, dose averaged linear energy transfer (LET) distribution and plan robustness, while fulfilling the set statistical conditions for reliable PG monitoring of selected spots. This opens the perspective of first delivering a small amount of selected spots of good detectability from the treatment plan, for measuring the beam range at few locations without extra dose exposure, prior to delivery of the entire fraction dose. Ongoing work aims at validating the proposed approach on more patient cases and tumor locations, taking also into account effects of inter-fractional anatomical changes.
Contact:
Prof. Dr. K. Parodi
References:
Tian L, Landry G, Dedes G, Kamp F, Pinto M, Niepel K, Belka C, Parodi K, Toward a new treatment planning approach accounting for in vivo proton range verification, Phys Med Biol. 2018;63:215025

