PET
Among the clinically most investigated techniques for monitoring proton or heavier ion treatment is positron emission tomography (PET), which relies on the detection of coincident 511 keV gamma pairs produced in the annihilation of positrons released in the decay of β+-emitters produced in nuclear interactions between the incoming ions and the traversed tissue. Compared to prompt gammas, this signal is intrinsically delayed with respect to the actual irradiation, depending on the half-life of the induced positron emitters, ranging from several milliseconds (e.g. 11 ms for 12N) up to tens of minutes (e.g. ca. 20 min for 11C). However, PET imaging can rely on proven technology intrinsically offering tomographic capabilities, in addition to the possibility of assessing additional physiological processes with externally injected tracers. Currently, the development of better and more cost-effective PET scanners for monitoring the small amount of irradiation-induced β+-activity is probably the most researched topic in the field of PET monitoring in particle therapy. Unfortunately, full exploitation of the corresponding signal in a clinical setting is a very often overlooked problem. In this regard, pre-calculation of the expected PET distribution is a requirement to allow the comparison between measurement and prediction, in order to assert the quality of the actually delivered treatment. The calculation of these predictions are nowadays very demanding in terms of computing resources, and the prediction for a single patient can take days depending on the computational infrastructure available. To address such an issue, Parodi and Bortfeld [1] proposed a fast approach that relies on simple mathematical principles. This approach started to be known as the filtering approach. Despite the simplicity, its building blocks can be quite challenging. To this end, in our group three further extensions of this method are being pursued:
- Integrating the prediction of the PET distribution following a proton therapy treatment in the treatment planning process [1];
- Enabling the prediction of the PET distribution induced by a carbon ion therapy treatment, which is more challenging than for protons due to the contribution of both projectile and target nuclear fragmentation reactions to the induced β+-activity [2];
- Enabling the assessment of the actual dose delivery from the acquired PET measurements in a carbon ion therapy treatment setting [3].
The first case applied to proton therapy, originally pursued in collaboration with the Heidelberg Ion Beam Therapy Center and MGH Boston[2], has been meanwhile successfully deployed in the research version of a commercially available treatment planning system (RayStation 5), i.e., the software responsible for the optimization of a radiotherapy treatment. An example can be seen in figure 1. This development will enable simple access to the predicted PET distributions shortly after the optimization of the treatment plan, without the need of cumbersome Monte Carlo simulations. Moreover, being directly integrated into the planning computational engine, the comparison of measured and predicted PET distributions can provide direct feedback on the validity of the computational models of the underlying treatment planning engine.
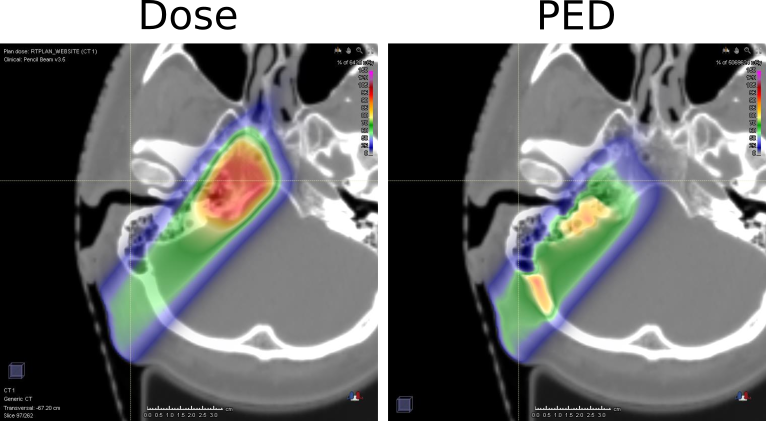
Figure 1.
The extension of this approach to carbon ion therapy is ongoing and includes dedicated solutions aiming to overcome the above-mentioned challenge of activity contributions from both projectile and target fragments, which violate the energy invariance assumption of the original filter formalism of [1]. On the other hand, research on the use of the filtering approach applied to carbon ions has been focused on predicting not only the PET distribution but also the delivered dose distribution from the PET measurement obtained with the in-vivo monitoring. The latter task would directly answer the main clinical need of devising information on the actually delivered dose, rather than evaluating less familiar PET distributions. An example of such dose deconvolution approach is depicted in figure 2.
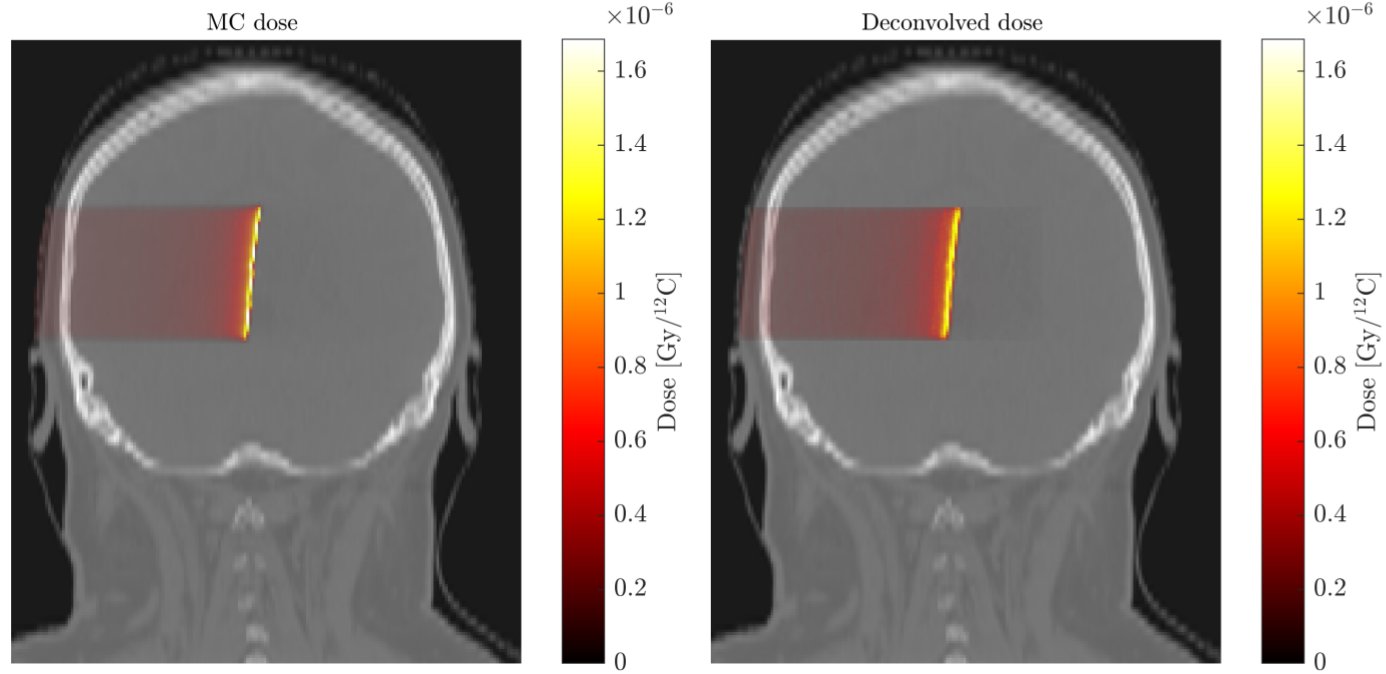
Figure 2.
Validation of all the developed computational tools is ongoing with both calculated and measured data, eventually aiming at integration in the treatment planning process, in close collaboration with RaySearch Laboratories and, in the case of carbon ion therapy, the Medical Imaging group at QST-NIRS in Chiba, Japan.
In addition to the computational developments, in the context of the project SIRMIO, we are aiming to develop a limited angle dual-head scanner for integration in a novel small animal proton irradiator.
Contact:
Dr. M. Pinto, Prof. Dr. K. Parodi
References:
[1] Parodi and Bortfeld, A filtering approach based on Gaussian–powerlaw convolutions for local PET verification of proton radiotherapy. Physics in Medicine & Biology, 51(8);2000:1991–2009.
[2] K. Frey, J. Bauer, D. Unholtz, C. Kurz, M. Krämer, T. Bortfeld, and K. Parodi. TPS PET – a TPS-based approach for in vivo dose verification with PET in proton therapy. Phys Med Biol 59(1); 2014:1
[3] A. Fochi, T. Hofmann, K. Parodi, M. Pinto. Positron emitter predictions for carbon ion therapy based on a filtering method, 56th Annual Conference of the Particle Therapy Co-Operative Group (PTCOG), 8-13 May 2017, Chiba, Japan.
[4] T. Hofmann, A. Fochi, M. Pinto, A. Mohammadi, M. Nitta, F. Nishikido, Y. Iwao, H. Tashima, E. Yoshida, M. Safavi-Naeni, A. Rosenfeld, T. Yamaya, K. Parodi. Dose reconstruction from PET images in carbon ion therapy: a deconvolution approach using an evolutionary algorithm, 2017 IEEE NSS/MIC, 21-28 October 2017, Atlanta (Georgia), USA.
Currently funded projects:

